Hypochlorite bleach is an oxidizing agent. There are two types of hypochlorite bleaching agent that are used in pulp and paper industries such as calcium hypochlorite and sodium hypochlorite. Generally, this stage is placed after chlorination and alkali extraction stages. It can be single stage or multiple stage process. Single stage hypochlorite bleaching process is one of the oldest bleaching processes. This bleaching agent oxidized some chromophoric groups of the lignin and decolorized it. Moreover, it solubilized the natural dyes, lignin and other impurities in the fiber. It is non selective type bleaching agent. It attacks not only the lignin but also cellulose, particularly if the reaction is not controlled properly. Calcium hypochlorite bleach is used in paper industry greatly compare to sodium hypochlorite. Possibly the following reactions are occurred in this stage.
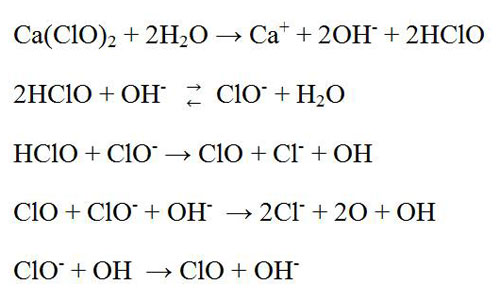
In 1880, hypochlorites are started using as a bleaching agent of wood pulp. Although several alternatives are originated that are environment friendly. They led to decreases the uses of it but still huge amount of hypochlorite bleach are used in many paper industries. Even, in United States and Canada due to cost effective, easy to make and uses. This bleach chemical is also used to remove of ink from recycled paper.
| Table of Contents: |
Factors for hypochlorite bleaching
The bleaching capacity is depends on some factor such as pH, temperature, retention time, consistency, nature of the pulp, chemical application etc.
Effect of pH
The hypochlorite bleaching action is greatly affected by the pH value. If the pH value becomes below 2 then Cl2 is the active ingredient, but at 4 to 7.5 the active ingredient is HOCl. Conversely at pH value over 8.5 the OCl − is the dominant species.
Consequently it is better to keep the pH between 9 to 11 at starting stage. The pH value up to 8 affects on the cellulose and degrades the fiber for the activities of the hypochlorous acid. During bleaching processes organic acid and carbon dioxide are formed. Therefore the pH value is decreasing. At the end point, it may below 7. This changing pH also causes the degradation of cellulose and fragile the fiber. It is also increases the consumption of bleach. To avoid this undesirable effect, it is recommended that use excess of alkali to neutralize the acid and CO2 formed during this bleaching stage. On the other hand too much higher pH value at starting stage can severely hindered the initial reaction rate.
Retention time
Retention time is an important factor for hypochlorite bleaching. The ideal condition for this stage is 1- 4 hours. Most of the bleaching plant maintains from 1 to 2 hours. Shorter time affects on the bleaching action while longer time may degrade the fiber. Over bleaching is always serious loss of strength.
Effect of temperature
Temperature is another important factor for this stage. The rate of hypochlorite bleach consumption will increase if you would not properly control the temperature. High temperature degrades the cellulose fiber but speed up the reaction and decreases the consumption. The optimum temperature depends upon several factors such as pulp consistency, pH and bleach ratio. Generally, it is better to keep 35-45 ⁰C, although the pulp properties are affected very little by the temperatures up to 60 ⁰C. It is better to heat the stock before the beach added. We should remember that the bleaching reaction is an exothermic reaction.
Effect of consistency
Higher consistency of stock increases the hypochlorite bleaching speed, increase production, reduce floor spaces, retention time, steam and bleach consumption. On the other hand, lower consistency increases retention time, steam and bleach consumption. Consequently, it is better to avoid lower consistency. The recommended consistency is from 10 to 15% for a continuous process. In case of high consistency agitator are desirable to break up fiber bundles and uniform mixing. But too much agitation may damage the fiber.
Nature of the pulp
Nature of the pulps is also responsible for hypochlorite bleaching capacity. Softwood pulps are relatively hard to bleach with hypochlorite compare to hardwood. Cooking process is another factor for pulp bleaching. In Sulfite pulping process the lignin residues are partly sulfonated and easily solubilized. Whereas, in kraft pulping process, lignin percentages are more and it is relatively less reactive, more condensed, and less reachable. Therefore Sulfite pulps are easier to bleach than sulfate pulps. Fresh kraft pulps are also readily bleaching compare to older kraft pulps.
Disadvantage
Hypochlorite bleach is not an environment friendly bleaching agent. Chloroform is produced in this stage which is released into environment. The specific reactions and conditions of chloroform formation are not established yet. Dichlorides and ethers are the main by-products in this reaction. Besides chloroform creation, the other significant weakness of this hypochlorite bleaching agent is that it greatly degrades the cellulose fiber.

sir,can u also elaborate rate of reaction???
i am working with a fiberboard production factory and would like to bleach pulp using calcium hypo chloride for the production of soft boards
what happens if we use caustic soda in the process of making calcium hypochloride. i mean to say is that while making calcium hypochloride, we have to pass chlorine in lime water. but if we add caustic soda in lime water and than pass chlorine to this solution what will happen.
and if we add cbs-x (tinopal from CIBA, Germany) in this process than this bleach will pulp of waste paper more brighly or not ?
I will try these chemicals in my paper industry.Thanks for your valuable information.
I need to know the recommend concentration of sodium hypochlorite bleaching agent and the addition ratio of it to fiber