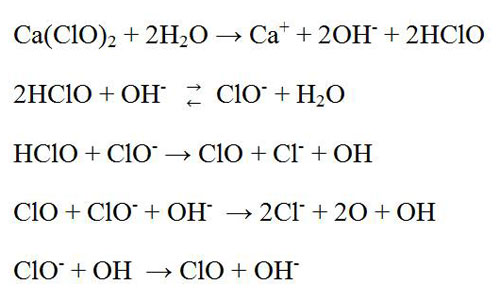Chlorine dioxide is an oxidizing agent for pulp bleaching. The chemical formula of this chlorine dioxide bleach is ClO2. It is a yellowish-green gas, bad odor and liquefied in 11⁰C which is red in color. It is an ECF (elemental chlorine-free) bleaching process which is currently most important bleaching technology in worldwide, especially for sulfate pulp. It is highly soluble in water, particularly in cold water. Chlorine dioxide bleaching is used in single or multi-stages process. Unlike chlorine, it does not react with water. It remains as a dissolved gas in water. It selectively attacks the phenolic groups of lignin without degrading cellulose fiber, allowing for increased yield and higher strength bleached pulp. For this property (selectivity), normally it is used in later bleaching stages when the lignin content is lower.
Commercially, it is first started in Sweden in 1946 for pulp bleaching. After 1950 it becomes a well established as bleaching agent for kraft pulp.
Effect of pH
The pH is a significant factor for chlorine dioxide bleaching. At high pH, it is less corrosive and rapidly reacts. A fraction of chlorine dioxide is converted to chlorite, chlorate or chloride. The overall reaction mechanisms are quite complex. The formation of chlorite increases with increasing of pH value whereas the formation of chlorate increases with decreasing the pH value, and the chloride ion is increased at a pH value below 3.4. The chlorite (ClO2–) exists as inert at above the ph 7. On the other hand this compound is very active below pH 7. However, the most efficient bleaching and maximum brightness are obtained in the first ClO2 stage (D0) at a pH level of about 3.5 – 4.0 while it is obtained in the second ClO2 stage (D1) at pH of 5.5 to 6. Normally, the pH varies from 7 to 3.5. So, less chlorine dioxide bleach is required for low pH than for high pH bleaching, but the temperature and retention time should be increased to speed up the reaction rate. At a lower pH (below 3.0) ClO2 can produced organic chlorine compounds which is not environment friendly.
It is possible to take advantage of the chlorite with maintaining a rapid rate of bleaching by keeping the pH level high at starting position and then lower. Corrosion is kept under control by using tile lined or acid proof brick towers and plastic-lined or ceramic pipe.
The residual ClO2 can create toxic fumes and corrosion at subsequent washing step. Therefore, SO2 or NaOH is used at the bottom of the tower to neutralizing it so that can be reduced corrosion and eliminated toxic fumes.
2ClO2 + 2NaOH → NaClO2 + NaClO3 + H2O
Retention time
The retention time may be as short as 15 to 60 minutes to complete the chlorine dioxide bleaching reactions. The average time maintains about two hours but some cases as much as 3 to 5 hours are allowed. The retention time over 3 hours may affect on the fiber. To control retention time some bleaching plants construct an up-flow tower followed by a bigger down-flow tower. Although some bleaching plants are carried out either up-flow or down-flow tower.
Effect of temperature
During the chlorine dioxide bleaching, generally, the temperature is maintained from 50 to 80⁰C. High temperatures can increases the reaction rate but above 80⁰C the strength of the fiber may be affected. The optimum temperature of this bleaching agent is 70-75⁰C.
Effect of consistency
The consistency of the pulp is very little effects on the reaction rate during chlorine dioxide bleaching. It is better to keep the stock consistency from 10 to 14%. High consistency increases the effectiveness of the bleaching. It reduces the consumption of the bleach, steam and the size of the towers. The ratio of the chlorine dioxide beach to pulp is not very important, except that above 0.6% on the pulp weight the brightness may be reduced slightly. The amount of ClO2 added to the pulp depends on the final brightness desired. Commercial, the amount of chlorine dioxide bleach usually consumed from 0.4 to 0.6 % for kraft pulps and 0.3 to 0.4 % for sulfite pulps.
Advantages
Chlorine dioxide is an ECF bleaching agent, which is used instead of elemental chlorine. The elemental chlorine bleaching has large environmental and health issues. It produces many toxic compounds as like organochlorine compounds, dioxins and furans, but the chlorine dioxide bleaching agent does not produce this chemicals. It is five to 10 times more soluble in water than chlorine.
Chlorine dioxide bleach is highly selective. It reacts rapidly with lignin without affect on cellulose fiber. It oxidizes, brighten and solubilize the lignin. Therefore the fiber achieve high brightness without pulp degradation; so, more strength, and more yield %.
The oxidizing power of ClO2 is two and a half times more than the oxidizing power of Cl2 on a mole per mole basis.
ClO2 + 2.5 H2 → HCl + H2O
The bleached pulps that are manufactured with using Chlorine dioxide bleach shows less tendency to revert in color than hypochlorite bleach.
Disadvantage
Chlorine dioxide bleaching agent is a very unstable compound, it is considered extremely toxic and explosive to handle and transport. At high concentration it is spontaneous explosive. Moreover, it is exploded by uv light, contact with mercury and organic matter, or by electric spark. Thus it is always produced on the plant site where it is used so that it could be applied immediatately after generation. It is expensive compare to calcium hypochlorite. To eliminate explosion, the chlorine dioxide bleach should protect from uv light, keep lower concentration, uncontaminated and cool. Moreover, to reduce consumption pH would to be controlled carefully.